Author: Denis Avetisyan
A new deep learning approach uses the brain’s own structural changes over time to forecast the progression of Alzheimer’s disease, potentially enabling earlier diagnosis and intervention.
This work introduces DATGN, a model utilizing deformation fields to generate temporal brain image sequences for improved Alzheimer’s disease prediction based on MRI scans.
Early diagnosis remains a critical challenge in managing Alzheimer’s disease, often hindered by the subtle and gradual nature of brain atrophy. This paper introduces a novel approach, ‘Deformation-aware Temporal Generation for Early Prediction of Alzheimers Disease’, which proposes a deep learning model-DATGN-to automatically learn and generate realistic temporal sequences of brain MRI images reflecting disease progression. By leveraging deformation fields and interpolating missing data, DATGN not only enhances the accuracy of future image prediction but also significantly improves Alzheimer’s disease classification when integrated with existing machine learning techniques. Could this method pave the way for more proactive and personalized interventions in combating this devastating disease?
The Imperative of Early Detection: A Foundation for Intervention
The clinical management of Alzheimer’s disease is profoundly complicated by the fact that diagnosis often occurs only after substantial and irreversible neuronal damage has already taken place. This late-stage detection significantly limits the effectiveness of potential interventions, as therapies aimed at slowing or halting disease progression are most promising when applied during the earliest phases of pathology. Consequently, individuals may receive a diagnosis when cognitive decline is readily apparent, but the underlying biological processes have been unfolding for years, even decades, rendering treatment less impactful. The challenge lies not only in identifying the disease but in pinpointing its presence before debilitating symptoms manifest, demanding a paradigm shift towards proactive and preventative strategies in neurological care.
Current diagnostic approaches for Alzheimer’s disease frequently encounter limitations due to their reliance on observable clinical symptoms and structural brain imaging conducted in later disease stages. By the time cognitive impairment is readily apparent – and detectable through standard assessments – significant and often irreversible neuronal damage has already occurred. Late-stage magnetic resonance imaging (MRI) can reveal widespread atrophy, but struggles to pinpoint the subtle, localized changes that precede these macroscopic effects. Consequently, interventions aimed at slowing or halting disease progression are often initiated after a critical threshold of neurological damage has been surpassed, diminishing their potential efficacy. This diagnostic delay underscores the urgent need for biomarkers and analytical techniques capable of identifying pathological processes years, even decades, before the onset of clinical symptoms.
The future of Alzheimer’s intervention hinges on a fundamental shift in diagnostic strategy, moving beyond reactive assessment of established damage to proactive monitoring of subtle structural changes within the brain. Longitudinal analysis, encompassing repeated brain scans over years, allows researchers to establish a baseline of individual brain structure and then track deviations that may precede clinical symptoms by a decade or more. This approach isn’t simply about detecting atrophy – the loss of brain tissue – but identifying patterns of change, such as alterations in the volume of the hippocampus or the thickness of the cortex, that serve as early warning signs. By charting these trajectories, clinicians can potentially predict which individuals are most likely to develop Alzheimer’s and intervene with preventative therapies before irreversible damage occurs, offering a critical window for slowing disease progression and preserving cognitive function.
Magnetic resonance imaging (MRI) offers an increasingly vital, non-invasive window into the evolving brain changes characteristic of Alzheimer’s disease. While conventional diagnostic approaches often detect structural alterations only after significant neuronal damage has occurred, MRI techniques can reveal subtle shifts in brain volume, cortical thickness, and white matter integrity years before clinical symptoms manifest. However, realizing the full potential of MRI for early detection demands sophisticated analytical tools capable of discerning these minute changes from normal variation and age-related decline. Advanced computational methods, including machine learning algorithms applied to longitudinal MRI data, are crucial for identifying predictive biomarkers and accurately tracking disease progression, ultimately paving the way for earlier interventions and more effective therapeutic strategies.

Precision in Preprocessing: Establishing a Foundation for Analysis
Rigorous preprocessing of MRI data is essential for minimizing artifacts and ensuring reliable analysis; a critical initial step is skull stripping, the removal of non-brain tissue. Methods such as HD-BET (Hybrid-Descriptive-BET) employ a deep learning approach to accurately segment the brain, even in the presence of imaging distortions or pathologies. HD-BET utilizes a hybrid architecture combining descriptive statistics and a convolutional neural network trained on a large, diverse dataset. This automated approach reduces the need for manual correction, improves efficiency, and enhances the precision of subsequent image analysis steps, including spatial normalization and volumetric measurements.
Spatial normalization to a standard atlas, most commonly the Montreal Neurological Institute 152 (MNI152) template, addresses inter-subject anatomical variability to enable meaningful comparisons of brain structure and function. This process involves applying a geometric transformation – typically a nonlinear algorithm – to each individual’s brain image, warping it to conform to the dimensions and geometry of the MNI152 space. Accurate normalization minimizes spurious differences attributable to anatomical variations, allowing researchers to confidently attribute observed effects to experimental manipulations or disease states. The MNI152 template is widely adopted due to its comprehensive representation of the human brain, its availability in standardized formats, and its compatibility with numerous neuroimaging software packages and databases.
The FSL (FMRIB Software Library) suite provides a comprehensive set of tools for MRI image preprocessing, with particular emphasis on orientation correction and spatial normalization. Orientation correction utilizes affine transformations to ensure all images are consistently aligned to a standard coordinate system, typically radiological convention. Spatial normalization, achieved through non-linear registration algorithms within FSL, warps individual subject images to fit a standard template, most commonly the MNI152 atlas. This process minimizes anatomical variation between subjects, enabling statistically valid group comparisons and longitudinal tracking of changes. FSL’s tools, including FLIRT for linear registration and FNIRT for non-linear registration, offer parameters for controlling the degree of warping and resampling methods to balance image preservation and alignment accuracy. The resulting normalized images are standardized in size and anatomical space, facilitating consistent data analysis across different scanners and subject populations.
Rigorous MRI preprocessing, encompassing steps like skull stripping and spatial normalization, is essential for reliable longitudinal analysis because it minimizes variability attributable to image acquisition and subject positioning. This standardization allows for the tracking of subtle changes within an individual over time, and facilitates the identification of patterns indicative of disease progression across a cohort. By reducing noise and aligning data to a common space, such as the MNI152 template, researchers can more confidently attribute observed changes to the underlying pathology rather than to technical artifacts. Accurate modeling of disease progression requires minimizing inter-subject and intra-subject variance, and effective preprocessing is a primary means of achieving this goal, ultimately enhancing the statistical power and interpretability of longitudinal studies.
Deformation Fields: A Geometric Foundation for Predictive Modeling
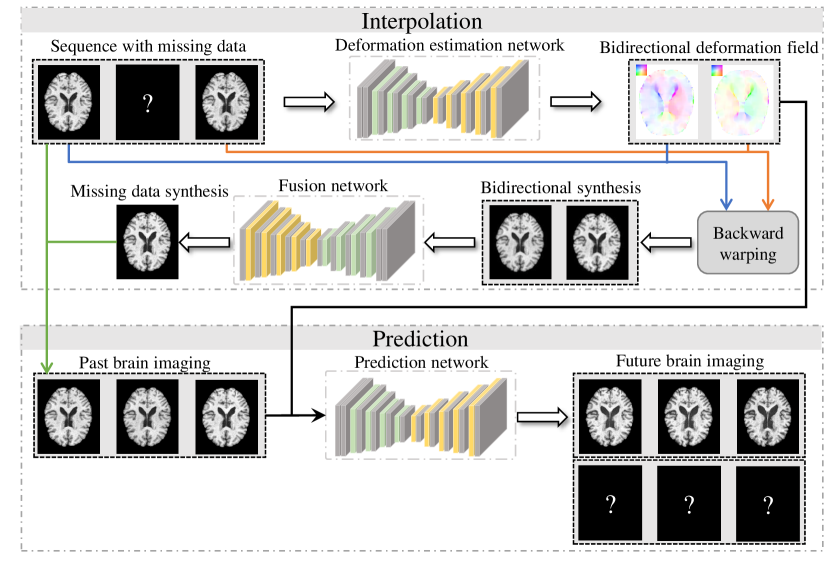
The DATGN model introduces a novel methodology for predicting future temporal Magnetic Resonance Imaging (MRI) data by directly incorporating deformation fields. These fields quantify changes in brain anatomy over time, representing the displacement of tissue from one time point to the next. Rather than relying solely on intensity-based information, DATGN utilizes these geometric changes as a primary input for prediction. This approach allows the model to account for structural variations that occur naturally with disease progression or normal aging, improving the accuracy of future state estimation. The deformation fields are computed using established image registration techniques and provide a spatially dense representation of anatomical change, effectively augmenting the temporal data with structural context.
The DATGN model mitigates the impact of missing data in temporal MRI sequences through the incorporation of bidirectional deformation fields. These fields quantify the displacement of anatomical structures between time points, allowing the model to infer data at missing intervals by warping and extrapolating from existing, valid data. Unlike methods that simply interpolate or ignore missing values, DATGN utilizes the spatial relationships defined by these deformation fields to reconstruct plausible anatomical configurations, thereby reducing prediction error. The bidirectional nature of the fields accounts for deformations occurring in both forward and reverse temporal directions, improving the accuracy of data imputation and subsequent sequence generation, particularly in scenarios with extended periods of missing data.
The DATGN model incorporates a modified Long Short-Term Memory network, termed DT-LSTM, to directly utilize bidirectional deformation fields for improved temporal prediction. Standard LSTMs process sequential data without explicit anatomical context; however, the DT-LSTM integrates deformation fields as additional input at each time step. These fields, representing the change in spatial configuration of the brain, modulate the hidden state updates within the LSTM, allowing the network to learn relationships between anatomical changes and future image states. This direct incorporation of deformation information enables the DT-LSTM to better capture complex temporal dependencies and produce more accurate predictions compared to conventional recurrent neural networks.
Quantitative evaluation demonstrates that the DATGN model consistently outperforms existing temporal generation models across both short- and long-term sequence generation tasks. Specifically, DATGN achieves higher Peak Signal-to-Noise Ratio (PSNR) values – indicating improved reconstruction quality – and correspondingly lower Mean Squared Error (MSE) values, signifying reduced prediction error. These metrics were consistently observed across multiple experimental datasets and evaluation horizons, establishing a statistically significant improvement in predictive performance compared to baseline models. The magnitude of improvement varied depending on the specific dataset and prediction length, but consistently favored DATGN in terms of both PSNR and MSE.
The DATGN model improves upon traditional recurrent neural networks (RNNs) by integrating anatomical information in the form of deformation fields, which quantify changes in brain morphology over time. Unlike standard RNNs that primarily process temporal data, DATGN directly incorporates these fields as input features, allowing the network to learn relationships between anatomical changes and future brain states. This direct incorporation of anatomical context enhances the model’s ability to generate more accurate and robust predictions, particularly when dealing with incomplete or noisy data, as the deformation fields provide a structural prior that constrains the solution space and reduces reliance solely on temporal dependencies.

Towards Proactive Care: Validation and the Promise of Personalized Prediction
Rigorous validation of the DATGN model against the Alzheimer’s Disease Neuroimaging Initiative (ADNI) dataset reveals a significant capacity for accurately predicting the progression of Alzheimer’s Disease. This assessment, utilizing a substantial collection of longitudinal MRI scans, demonstrates the model’s ability to discern subtle patterns of brain change indicative of disease development. The findings suggest DATGN effectively captures the nuanced deterioration in Gray Matter Volume that often precedes clinical symptoms, offering a promising avenue for early detection and intervention. This predictive capability holds the potential to shift the paradigm of Alzheimer’s management, moving towards proactive, personalized strategies designed to slow or even prevent disease advancement.
The model’s strength lies in its ability to discern nuanced alterations in brain structure by analyzing longitudinal magnetic resonance imaging (MRI) data. Rather than simply measuring Gray Matter Volume at a single point in time, the approach incorporates deformation fields – maps detailing how the brain changes shape over months or years. These fields reveal subtle tissue loss or atrophy often preceding overt symptoms of Alzheimer’s Disease. By tracking these deformations, the model effectively amplifies the signal of early pathological changes, allowing for the detection of disease progression long before traditional volumetric analyses might reveal them. This sensitivity to early indicators suggests a powerful tool for proactive monitoring and potential intervention before irreversible damage occurs.
The study demonstrated that augmenting existing datasets with synthetically generated MRI scans, created using the DATGN model, significantly boosted the accuracy of Alzheimer’s Disease classification. Specifically, incorporating this artificial data improved the model’s ability to differentiate between patients with Alzheimer’s Disease and cognitively normal individuals, as well as to distinguish between Alzheimer’s patients, those with Mild Cognitive Impairment, and healthy controls. This suggests that DATGN not only models disease progression but also effectively expands the training data available, particularly valuable when dealing with the limited size and cost of acquiring longitudinal neuroimaging data. The enhanced classification performance highlights the potential of synthetic data to overcome data scarcity challenges and refine diagnostic algorithms for earlier and more accurate detection of Alzheimer’s Disease.
The capacity to forecast future brain states, as demonstrated by this research, represents a significant step towards proactive Alzheimer’s disease management. By anticipating neuroanatomical changes before the onset of clinical symptoms, the model offers the potential for earlier and more accurate diagnoses, shifting the paradigm from reactive treatment to preventative intervention. This predictive capability extends beyond diagnosis, paving the way for personalized treatment strategies tailored to an individual’s projected disease trajectory. Interventions could be initiated at a pre-symptomatic stage, potentially slowing disease progression or even delaying onset, and allowing clinicians to optimize therapeutic approaches based on forecasted brain changes rather than solely on current clinical presentation. Ultimately, this work suggests a future where neurological care is not only responsive but anticipatory, enhancing patient outcomes and quality of life.
Continued development of the DATGN model prioritizes enhanced precision and broader clinical applicability. Researchers aim to refine the model’s architecture and training methodologies, seeking to improve its ability to discern subtle indicators of Alzheimer’s Disease progression. A key focus involves incorporating a wider range of biomarkers – including cerebrospinal fluid data, genetic information, and tau-PET imaging – to create a more holistic and informative predictive framework. Furthermore, studies are planned to evaluate the model’s performance across diverse patient populations, accounting for variations in ethnicity, age, and lifestyle, ultimately striving for a universally effective tool for early diagnosis and personalized treatment planning in Alzheimer’s disease.

The pursuit of accurate Alzheimer’s disease prediction, as detailed in this work, demands a commitment to foundational mathematical principles. The DATGN model, with its emphasis on deformation fields for temporal image generation, exemplifies this rigor. As Andrew Ng once stated, “Machine learning is about making predictions based on data.” This deceptively simple statement underscores the necessity of a provably correct underlying mechanism. DATGN isn’t simply attempting to correlate image changes with disease progression; it models the deformation process itself, offering a more elegant and reliable foundation for early diagnosis. Redundancy in model design introduces potential for abstraction leaks; thus, DATGN’s focus on minimal, mathematically sound deformation fields represents a step toward true algorithmic purity.
Future Directions
The presented work, while demonstrating a capacity for temporal image synthesis, merely skirts the edges of a truly predictive model. The deformation fields, crucial as they are, remain descriptive-snapshots of change rather than harbingers of it. A rigorous formulation would demand a derivation of these fields from first principles, linked directly to the underlying pathophysiology of Alzheimer’s disease. Currently, the model extrapolates; a provable solution would necessitate deduction.
Further exploration must address the inherent limitations in equating image similarity with disease progression. While morphological changes are correlated with Alzheimer’s, they are not the disease itself. The signal, however precisely interpolated, remains a proxy. A truly elegant solution would integrate multi-modal data-genetic markers, cerebrospinal fluid analysis, cognitive assessments-all formally linked within a unified mathematical framework. Such integration is not merely a matter of concatenation, but of establishing invariant relationships.
Ultimately, the pursuit of early diagnosis is not about achieving incrementally higher accuracy on benchmark datasets. It is about constructing a model that embodies a fundamental understanding of neurodegeneration. Until the deformation fields are derived, not observed, and the model predicts with logical necessity, not statistical probability, it remains a sophisticated approximation-a beautifully rendered shadow of the truth.
Original article: https://arxiv.org/pdf/2511.21114.pdf
Contact the author: https://www.linkedin.com/in/avetisyan/
See also:
- 2025 Crypto Wallets: Secure, Smart, and Surprisingly Simple!
- Gold Rate Forecast
- Brown Dust 2 Mirror Wars (PvP) Tier List – July 2025
- Banks & Shadows: A 2026 Outlook
- Gemini’s Execs Vanish Like Ghosts-Crypto’s Latest Drama!
- ETH PREDICTION. ETH cryptocurrency
- The 10 Most Beautiful Women in the World for 2026, According to the Golden Ratio
- The Weight of Choice: Chipotle and Dutch Bros
- Uncovering Hidden Groups: A New Approach to Social Network Analysis
- Gay Actors Who Are Notoriously Private About Their Lives
2025-11-28 06:04